PROGREAT ALPHA™ Peripheral Microcatheter
PRODUCT OVERVIEW

PROGREAT ALPHA™ 2.0 Fr designed to enhance access to small peripheral vessels1,2
- Low profile distal OD—smallest microcatheter specifically for peripheral vasculature1,2
- TERUMO glide technology for navigation through tortuous anatomy1,2

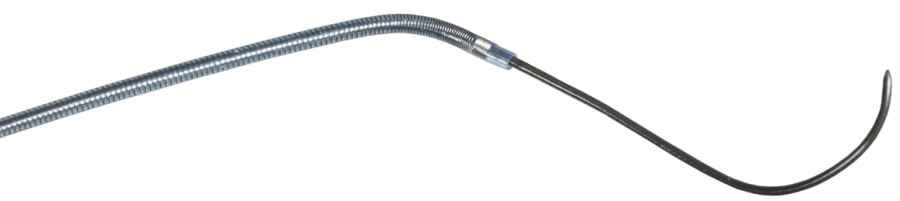
Available in Pre-Shaped Tips
CONFIDENTLY DELIVER EMBOLOTHERAPY
- Thin-wall design with large 0.019" lumen¹,²
- Tungsten coil reinforcement for kink resistance³
- DMSO compatible⁴

IMPROVE THE EFFICIENCY OF YOUR EMBOLIZATION PROCEDURES
- Supports the delivery of large embolics—up to 500 μm spherical particles, and the AZUR® CX 0.018” Peripheral Coil System²
- Designed to provide clear images, with superior PSI, compared to similar-sized microcatheters¹,²
DESIGNED FOR SMALL VESSEL EMBOLIZATION PROCEDURES SUCH AS:
- Prostate artery embolization (PAE)
- Pre Y-90 embolization
- Gastrointestinal bleeds
PRODUCT CODES
PROGREAT ALPHA™ Ordering Information
| Fr Size | Product Code | Length (cm) | Tip Shape | Max Pressure (psi) | RO Markers | Hydrophilic Coating Length (cm) |
| 2.0 | MC*PC2011Y | 110 | Straight | 750 | 1 | 50 |
| MC*PC2013Y | 130 | Straight | 1 | 70 | ||
| MC*PC2013ZRA | 130 | 70 Deg. Angle | 1 | 70 | ||
| MC*PC2013ZRC | 130 | J Curve | 1 | 70 | ||
| MC*PC2015Y | 150 | Straight | 1 | 90 | ||
| MC*PC2015ZRA | 150 | 70 Deg. Angle | 1 | 90 | ||
| MC*PC2015ZRC | 150 | J Curve | 1 | 90 |
PRODUCT SPECIFICATIONS
PROGREAT ALPHA™ Specifications
| Catheter OD Distal/Proximal (Fr/mm) |
LENGTH (cm) |
INNER DIAMETER (in/mm) |
MAX GUIDEWIRE (in) |
EMBOLIC COMPATIBILITY |
DEAD SPACE VOLUME (mL) |
ACTUAL FLOW RATE* (mL/sec) 750 psi |
| 2.0/2.7 Fr (0.67/0.90mm) |
110 | 0.019/0.49 | 0.016 | AZUR® CX 0.018” Coils/Microspheres up to 500m |
0.28 | 0.9 |
| 2.0/2.7 Fr (0.67/0.90mm) |
130 | 0.019/0.49 | 0.016 | AZUR® CX 0.018” Coils/Microspheres up to 500m |
0.32 | 0.8 |
| 2.0/2.7 Fr (0.67/0.90mm) |
150 | 0.019/0.49 | 0.016 | AZUR® CX 0.018” Coils/Microspheres up to 500m |
0.38 | 0.6 |
RX ONLY. The advertisement is directed to physicians only, and not to consumers. Refer to product labels and packaging insert for complete warnings, precautions, potential complications, and instructions for use. Products may not have regulatory approval in all countries. Please contact your local sales representative if you have questions about the availability of products in your area.
REFERENCES
- Progreat Microcatheters IFU PG34E002-05 Rev.10 Revised 2023-11
- Data on File. Terumo Medical Corporation.
- PROGREAT Catheter 510(k); 2003.
- PROGREAT DMSO Compatibility Statement Letter.



