PROGREAT® Microcatheters
PRODUCT OVERVIEW
PROGREAT® Microcatheters are designed to allow navigation through tortuous peripheral vessels for optimal access and delivery of therapeutic embolization.


DESIGNED TO CONFIDENTLY DELIVER EMBOLOTHERAPY
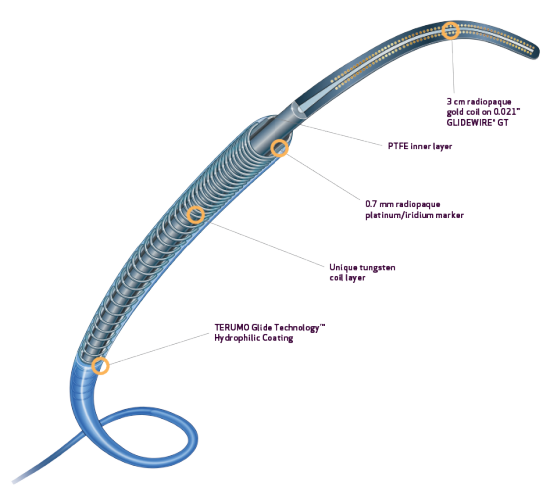
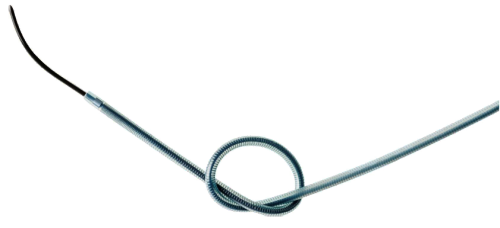
- Tungsten coil reinforcement for kink resistance2
- PTFE inner layer is designed for exceptional guidewire tracking, with virtually frictionless delivery of coils and other embolics2
- DMSO Compatible3
- Radiopaque 0.7 mm platinum/iridium markers allow for rapid and precise positioning4

Available in Pre-Shaped Tips
DESIGNED TO ENHANCE ACCESS TO SMALL PERIPHERAL VESSELS
- TERUMO Glide TechnologyTM hydrophilic coating enhances navigation through tortuous anatomy1,2
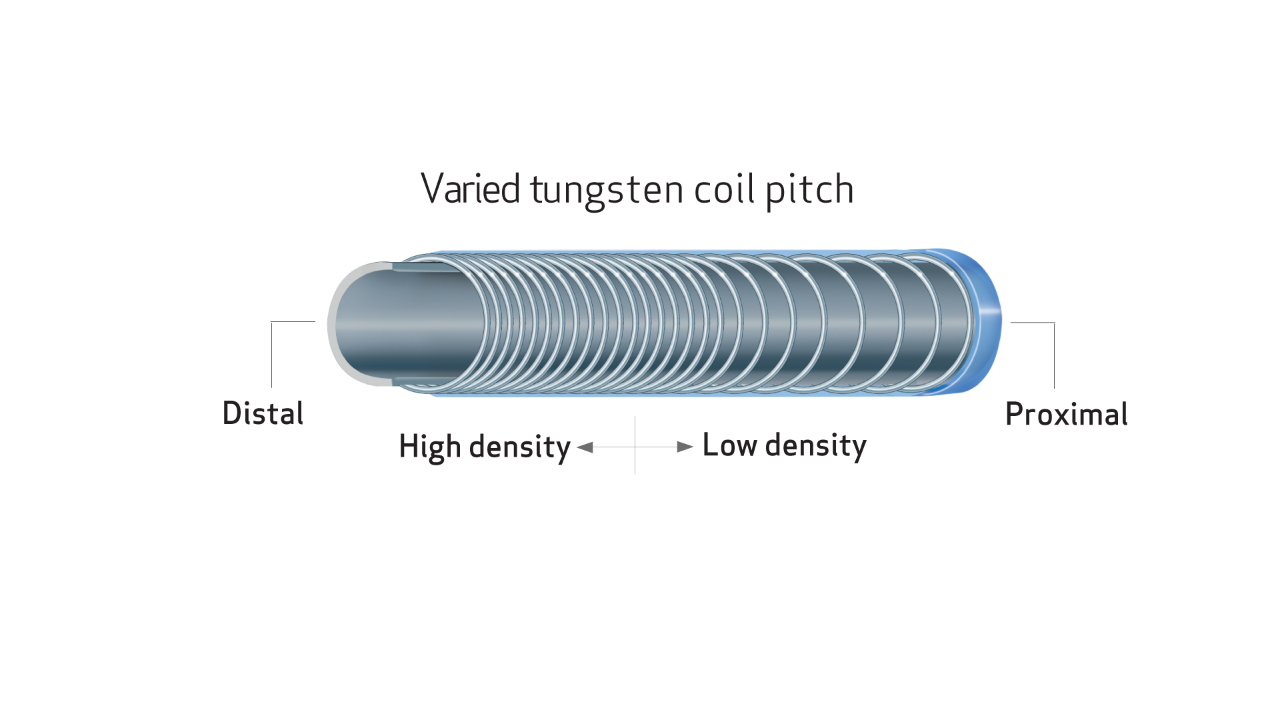
- Unique varied tungsten coil pitch construction provides distal flexibility and proximal pushability, enhancing vessel selectivity and catheter placement
PROGREAT® COAXIAL MICROCATHETER SYSTEM
- Preloaded with uniquely designed 0.021” GLIDEWIRE® GT for the 2.8Fr system and 0.018" GLIDEWIRE® GT for the 2.4Fr system1
- Available in 2.8Fr and 2.4Fr
- Enables simultaneous preparation of microcatheter and guidewire, which may save time and increase procedural efficiency1
PROGREAT 2.4 COAXIAL PROVEN TO REACH DISTAL VESSELS WITH EASE⁴
- Provides greater vessel selectivity and demonstrated superiority in traversing tortuous anatomy
- Provides best-in-class kink resistance, demonstrated in both the distal and proximal shaft
PRODUCT CODES
PROGREAT® Microcatheter Product Codes
Includes hemostatic valve and shaping mandrel
| Fr Size | Product Code | Length (cm) | Tip Shape | Max Pressure (psi) | RO Markers | Hydrophilic Coating Length (cm) |
| 2.4 | MC*PB2411Y | 110 | Straight | 750 | 1 | 50 |
| MC*PB2413Y | 130 | Straight | 1 | 70 | ||
| MC*PB2413ZRA | 130 | 70 Deg. Angle | 1 | 70 | ||
| MC*PB2413ZRC | 130 | J Curve | 1 | 70 | ||
| MC*PV2415Y | 150 | Straight | 2 | 90 | ||
| MC*PV2415ZRA | 150 | 70 Deg. Angle | 2 | 90 | ||
| MC*PV2415ZRC | 150 | J Curve | 2 | 90 | ||
| 2.7 | MC*PC2711Y | 110 | Straight | 750 | - | 50 |
| MC*PC2713Y | 130 | Straight | - | 70 | ||
| 2.8 | MC*PB2811Y | 110 | Straight | 900 | 1 | 50 |
| MC*PB2813Y | 130 | Straight | 1 | 70 | ||
| MC*PV2815Y | 150 | Straight | 2 | 90 |
PROGREAT® Coaxial Microcatheter System Codes
Includes unique GLIDEWIRE® GT Guidewire, wire stopper, guidewire introducer, hemostatic valve, 2.5 mL syringe, and shaping mandrel
| Fr Size | Product Code |
Length (cm) |
Max Pressure (PSI) |
RO Markers | Hydrophilic Coating Length (cm) |
Glidewire® GT Length (cm) |
Glidewire® GT Size (in) |
| 2.4 Fr | MC*PE24111YB | 110 | 750 | 1 | 50 | 120 | 0.018 |
| 2.4 Fr | MC*PE24131YB | 130 | 750 | 1 | 70 | 140 | 0.018 |
| 2.4 Fr | MC*PE24151YV | 150 | 750 | 2 | 90 | 160 | 0.018 |
| 2.7 Fr | MC*PE27111Y | 110 | 750 | - | 50 | 120 | 0.021 |
| 2.7 Fr | MC*PE27131Y | 130 | 750 | - | 70 | 140 | 0.021 |
| 2.8 Fr | MC*PE28111YB | 110 | 900 | 1 | 50 | 120 | 0.021 |
| 2.8 Fr | MC*PE28131YB | 130 | 900 | 1 | 70 | 140 | 0.021 |
| 2.8 Fr | MC*PE28151YV | 150 | 900 | 2 | 90 | 160 | 0.021 |
PRODUCT SPECIFICATIONS
| Catheter OD | Length (cm) |
Inner Diameter (in/mm) |
Max GW (in) |
Embolic Compatibility |
Dead Space Volume (mL) |
Actual Flow Rate* (mL/sec) @ 750 psi | Actual Flow Rate* (mL/sec) @ 900 psi |
| 2.4/2.9Fr (0.80/0.97mm) |
110 | 0.022"/0.57 | 0.018" | 0.018" Coils/Microspheres up to 700m | 0.38 | 2.0 | - |
| 2.4/2.9Fr (0.80/0.97mm) |
130 | 0.022"/0.57 | 0.018" | 0.018" Coils/Microspheres up to 700m | 0.43 | 1.7 | - |
| 2.4/2.9Fr (0.80/0.97mm) |
150 | 0.022"/0.57 | 0.018" | 0.018" Coils/Microspheres up to 700m | 0.47 | 1.4 | - |
| 2.7/2.9Fr (0.90/0.97mm) |
110 | 0.025"/0.065 | 0.021" | 0.018" Coils/Microspheres up to 800m | 0.46 | 3.0 | - |
| 2.7/2.9Fr (0.90/0.97mm) |
130 | 0.025"/0.065 | 0.021" | 0.018" Coils/Microspheres up to 800m | 0.53 | 2.6 | - |
| 2.8/3.0Fr (0.93/1.00mm) |
110 | 0.027"/0.70 | 0.021" | 0.018" Coils/Microspheres up to 900m | 0.53 | 3.5 | 3.9 |
| 2.8/3.0Fr (0.93/1.00mm) |
130 | 0.027"/0.70 | 0.021" | 0.018" Coils/Microspheres up to 900m | 0.59 | 3.0 | 3.6 |
| 2.8/3.0Fr (0.93/1.00mm) |
150 | 0.027"/0.70 | 0.021" | 0.018" Coils/Microspheres up to 900m | 0.66 | 2.7 | 3.2 |
RX ONLY. The advertisement is directed to physicians only, and not to consumers. Refer to product labels and packaging insert for complete warnings, precautions, potential complications, and instructions for use. Products may not have regulatory approval in all countries. Please contact your local sales representative if you have questions about the availability of products in your area.
REFERENCES
- Progreat Microcatheters IFU PG34E002-05 Rev.10 Revised 2023-11
- PROGREAT Catheter 510(k); 2003
- PROGREAT DMSO Compatibility Statement Letter.
- Data on file



